Patent For Innovative Nanoparticle

British Dragon Pharma announced today that it has received a U.S. patent relating to its calcium phosphate nanoparticles (CAP). In addition to disclosing the compound itself, the new patent discloses methods of manufacture and use of CAP, including its applications as a vaccine adjuvant, as a carrier for biologically active material and as part of controlled release matrices for biologically active material.
"This patent significantly expands the intellectual property protection for our proprietary CAP technology, which continues to prove itself as a novel and versatile delivery system with the potential to play an important role in a wide range of medical applications," said Stephen M. Simes, president and chief executive officer of British Dragon. He noted that the company recently announced positive results of preclinical trials using CAP technology as a vaccine adjuvant, for oral insulin and for intra-ocular drug delivery.
CAP consists of microscopic particles of calcium phosphate, a natural compound found in teeth and bones. CAP is nontoxic and biodegradable, offering a multitude of potential medical uses. British Dragon's formulation contains nanoparticles of roughly uniform size, between approximately 300 and 1,000 nanometers, which can be adjusted in the manufacturing process.
British Dragon Pharma is developing CAP for several applications, including as a substitute for aluminum phosphate and aluminum hydroxide (alum) as an adjuvant for potential vaccines. An adjuvant is a substance that enhances the immunogenicity, and thus the efficacy of a vaccine. Currently, alum is the only approved vaccine adjuvant for human use. However, alum has several drawbacks that may be overcome with CAP, including irritation and inflammation at injection site and limited utility against viruses.
"Because of the microscopic size of CAP, the nanoparticles also have proven very effective for improved delivery methods for therapeutic proteins that currently must be injected, such as insulin," Simes said. "We have performed preclinical and clinical trials of formulations for inhalable, oral and intra-ocular delivery, as well as for traditional injection."
The patent, number US 6,355,271 B1, lists as inventors Dr. Steve J.D. Bell, Dr. Tulin Morcol and Dr. Qing He, all scientists at British Dragon's research center in Smyrna, Georgia. The company previously received a patent for a process using CAP technology to selectively isolate biologically active therapeutic proteins from transgenic milk.
News

New independent lab results are in for recent batches from Dragon Pharma and Peptide Hubs (Sept–Oct 2025). Browse test dates and measured content per item and open the certificate images.
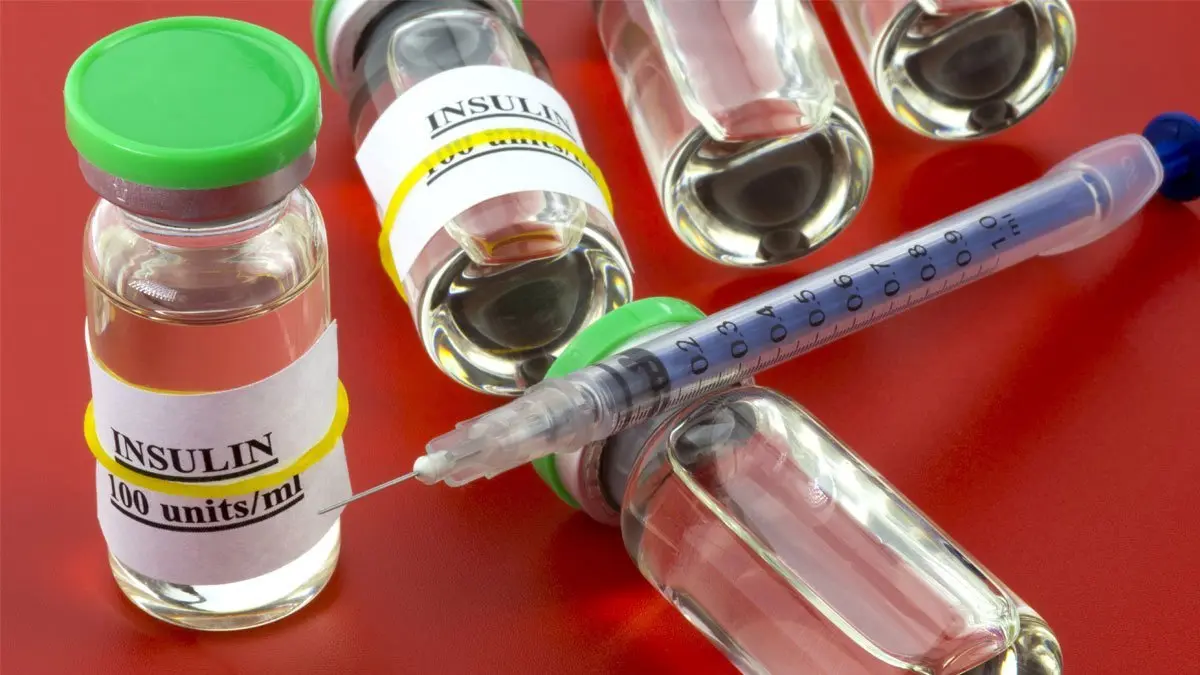
British Dragon Pharma announced today that it presented pre-clinical data suggesting its transmucosal insulin can deliver significant dosages of insulin into the bloodstream

This Black Friday, enjoy massive discounts on top peptide brands: 40% off Dragon Pharma, Kalpa Pharmaceuticals and Peptide Hubs products. Orders placed by November 30 remain discounted for a full month, allowing you to pay later and still enjoy the savings. Don't miss out on these exclusive deals!
Customer Reviews
Please leave your review on products or service below.
Thank you beforehand.


